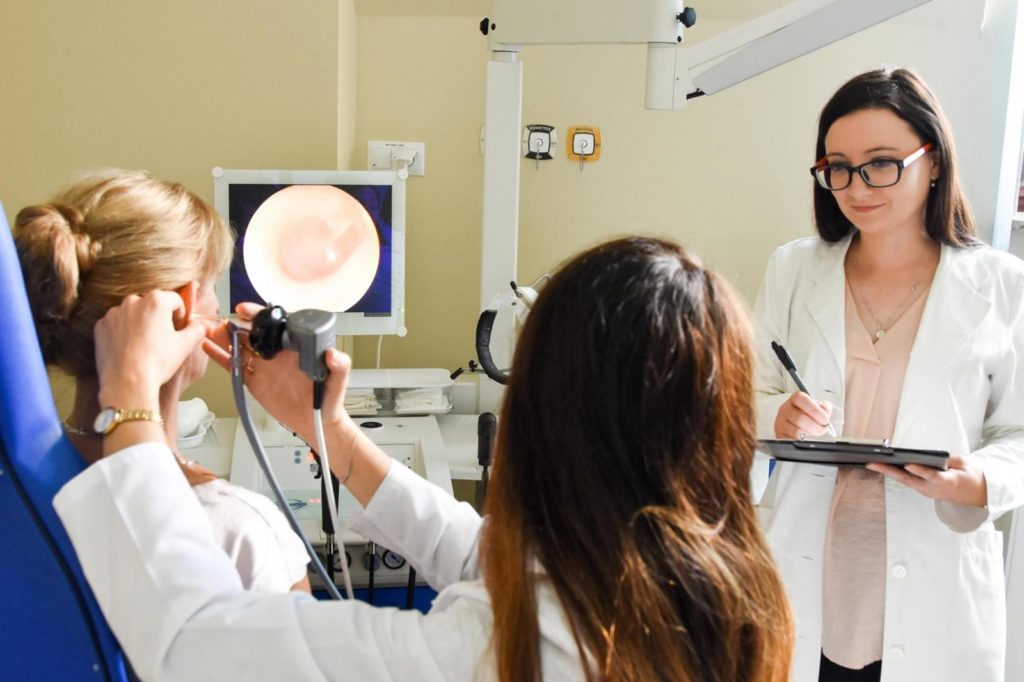
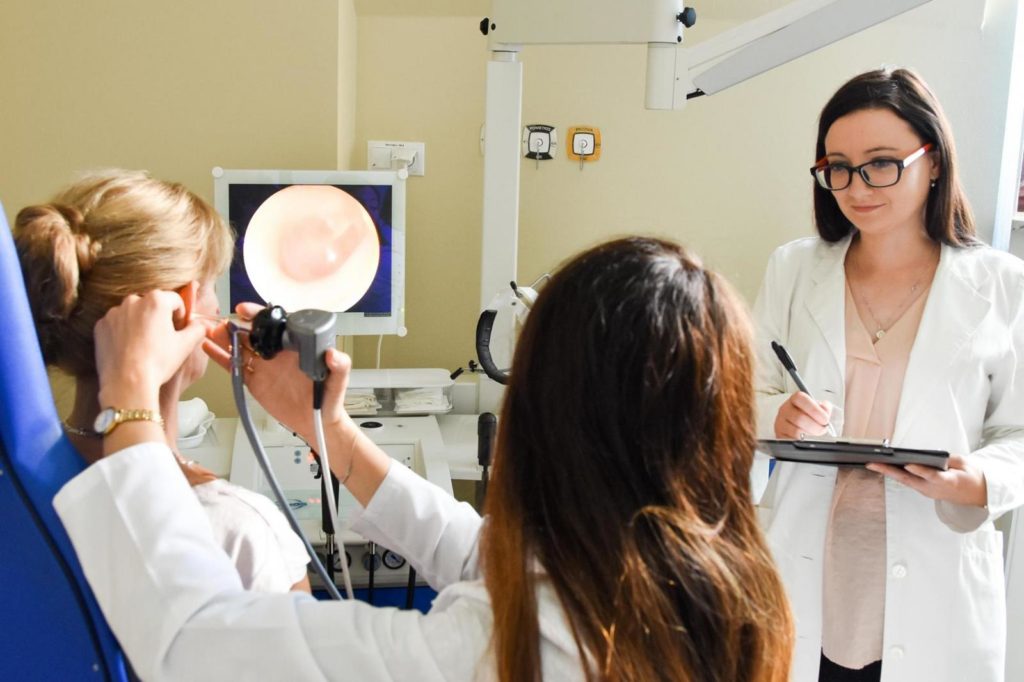
Prof. Piotr H. Skarzynski, MD, PhD, MSc finished three specializations: otorhinolaryngology, pediatric otorhinolaryngology and audiology and phoniatrics. He is realizing specialization in public health (since 2020). He is a member of 32 scientific societies. He participated in the 3rd Stakeholders Consultation meeting during which the World Hearing Forum of WHO was announced.
He is also a member of Consultant Committee of International Experts of CPAM-VBMS (for special invitation), a Honorary Member of ORL Danube Society, and a member of the Roster of Experts on Digital Health of WHO. He is also a Vice Chairman of Junior European Rhinology Society (2010–2014), Member of Board (2014–2016), Member of Congress and Meeting Department of European Academy of Otology and Neuro-Otology, Representative Board Member (till 2019) and Vice-President and Institutional Representative (since 2019) of International Society for Telemedicine and e-Health, Regional Representative of Europe of International Society of Audiology and Board Secretary of the Society of Otorhinolaryngologists, Phoniatrists and Audiologists. He is also a Vice-President of Hearring Group, a Member of Hearing Committee of American Academy of Otorhinolaryngology-Head and Neck Surgery and an Auditor of European Federation of Audiology Societies. He is also a Member of the State Examination Committee of Poland in the field of otorhinolaryngology, Expert Witness of Laryngology of Provincial Court of Warsaw (Warszawa-Praga; until 2018), Delegate for Regional Assembly of Regional Medical Chamber in Warsaw, a member of Young Scientists Council of Medical University of Warsaw and a Member of Scientific Council of the Institute of Physiology and Pathology of Hearing. Moreover, he is a member of Policy Council of Audiophonology at the Medical University of Warsaw, a candidate for an expert of Smart Growth Operational Programme 2014-2020 (The National Centre for Research and Development), a member of the University Council of the Maria Grzegorzewska University, a member of the Policy Council of „Health of Poles” Congress and a member of the Team of Experts to develop and update the program of specialization in the field of pediatric otorhinolaryngology at the Medical Center of Postgraduate Education. He is an active participant of many conferences – over 1869 presentations (117 as an Invited Speaker, 128 round tables, 140 courses as an Instructor), 957 publications (IF – 226,681, IC – 48781,04, scientific points of Ministry of Science and Higher Education – 11822). His papers are cited by national and international researchers (1207 according to Scopus, 2262 acc. to Google Scholar, h-index 15 acc. to Scopus and Web of Science, 21 acc. to Google Scholar). He is also an Associate Editor of Journal of Hearing Science and an Nowa Audiofonologia. He is a reviewer of 44 national and international scientific journals.
He executes numerous national and international projects connected with telemedicine, e-health, including teleconsultations, telerehabilitation and telefitting in the numerous European, Asian and African countries. He coordinates and manages the multifaceted telefitting procedures on the frame of International Centre of Hearing and Speech Medincus, collaborating with agencies in Poland and abroad. He is also a member of researching and working group of Nationwide Network of Teleaudiology (NNT).
Research interests: ENT surgery, otology, rhinology, hearing implantable devices and hearing aids, telemedicine and e-Health, robotics, tinnitus, hearing screening in Europe, Africa, Asia and South America.